=
Mycoplasma pneumoniae: The Covert Operatives in Biofilm Combat
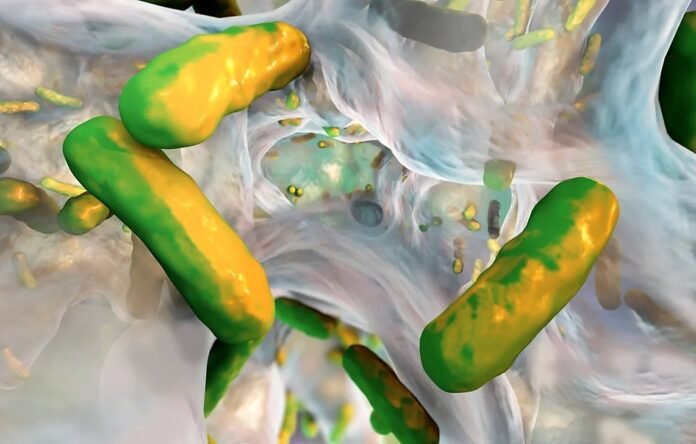
In an astonishing feat of scientific ingenuity, researchers have successfully transformed Mycoplasma pneumoniae, a notorious bacterium responsible for lung infections, into a formidable “double agent.” By employing genetic modifications, these scientists have orchestrated a targeted assault on the biofilms engineered by the insidious pathogen Pseudomonas aeruginosa. This groundbreaking study, documented in the prestigious journal Nature Biotechnology, unravels the untapped therapeutic potential of live bacteria, marking a historic milestone in the utilization of Mycoplasma for medical purposes.
Decoding the Enigma: Biofilms and the Versatility of Live Bacteria
Biofilms, enigmatic communities of bacteria that flourish in various bodily domains like the lungs or catheters, pose a formidable challenge due to their robust resistance to traditional antimicrobial therapies. To counter this formidable adversary, researchers have turned their attention to live bacteria as a potential panacea. Unlike their minuscule molecular counterparts, live bacteria possess the uncanny ability to execute multifaceted tasks and can be genetically engineered to produce an array of proteins concurrently. This innovative approach presents a fresh repertoire of possibilities to target diseases and mechanisms that have long evaded the grasp of conventional treatments.
Unleashing Engineered Mycoplasma for the Battle against Lung Diseases
At the helm of this revolutionary endeavor is Maria Lluch-Senar, an esteemed biotechnologist from the University of Catalonia and the visionary founder of Pulmobiotics. Her audacious mission revolves around engineering bacteria capable of combating lung diseases, with a particular focus on the perils of ventilator-associated pneumonia, often orchestrated by the notorious Pseudomonas aeruginosa. Mycoplasma pneumoniae, selected for its genetic stability that prevents recombination and ensures a confined modification, emerged as an ideal candidate for the groundbreaking genetic manipulations. Furthermore, its compact genome and the absence of a cell wall significantly reduce the risk of immune backlash.
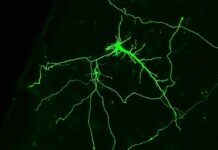
Through an intricate process, the team meticulously extracted pathogenic genes from Mycoplasma pneumoniae to guarantee its safety, subsequently introducing sets of genomes into the commonly employed bacterium, Escherichia coli, renowned for its therapeutic applications. These introduced genomes harbored the genetic blueprints for manufacturing enzymes capable of dismantling biofilms and a potent toxin to exterminate the treacherous Pseudomonas aeruginosa. Eventually, these coveted genes were transplanted into Mycoplasma pneumoniae. Overcoming the sluggish growth rate of the bacterium, the resilient team persevered through arduous years of testing, finally poised to evaluate the efficacy of the modified bacteria within the intricate battleground of mice.
The Dawn of Triumph: Promising Results and a Glimpse into Future Prospects
Subjecting mice to the wrath of Pseudomonas aeruginosa infection, the scientists unleashed the engineered Mycoplasma pneumoniae upon some of the afflicted rodents, while leaving others untreated. The outcome illuminated a resounding triumph: mice treated with the modified bacteria displayed significantly milder lung lesions after a mere two days, prolonging their survival by an average of seven days post-infection. In stark contrast, the untreated mice faced exacerbated lesions and a meager survival time of a mere two days. The researchers further tested the bacteria on biofilm-laden endotracheal tubes obtained from patients suffering from ventilator-associated pneumonia. Astonishingly, within a mere 24 hours, the tubes treated with the engineered bacteria showcased significantly reduced Pseudomonas aeruginosa loads compared to those subjected solely to antibiotic treatment or left untreated, highlighting the remarkable potential of this groundbreaking approach.
While the engineered bacteria may not offer a complete panacea, Maria Lluch-Senar expressed her profound satisfaction with the results, emphasizing the inherent unpredictability that accompanies scientific research. Looking ahead, she envisions propelling these engineered bacteria into clinical applications, envisaging their transformative potential in the realm of human pneumonia treatment. Intriguingly, the applications of these engineered bacteria extend far beyond their efficacy in lung diseases. The scientific community, spurred by these groundbreaking findings, eagerly anticipates the exploration of broader horizons, envisioning a cascade of potential applications across a diverse range of ailments.
Expanding the boundaries of scientific discovery, Lluch-Senar intends to apply her pioneering engineering techniques to combat other respiratory maladies, including lung cancer and asthma. With a steadfast commitment to translating these unprecedented advancements into tangible solutions, her vision remains firmly fixed on the noble pursuit of enhancing societal well-being and bolstering the overall health of individuals.
Unveiling a Future of Possibilities and Untapped Potential
The resounding success achieved by genetically engineered Mycoplasma pneumoniae in dismantling biofilms within a mouse model of ventilator-associated pneumonia holds profound implications for the treatment of various lung diseases. This revolutionary approach transcends the confines of traditional therapies, breathing new life into the battle against biofilm-related infections.
The adoption of live bacteria as therapeutic agents heralds a paradigm shift in the field of medicine, particularly concerning the treatment of infections linked to biofilms. The ability to engineer bacteria to produce tailored proteins and enzymes imparts a level of precision and intricacy that eludes traditional small molecule drugs. This, in turn, unlocks a vast array of possibilities for developing targeted treatments for previously enigmatic diseases and mechanisms that once defied our understanding.
Moreover, the study’s findings tantalize the scientific community with the boundless potential of engineered bacteria beyond the realm of lung diseases. Experts like Dave Hava eagerly anticipate the exploration of this innovative platform’s broader scope and its prospective impact on a myriad of conditions. By harnessing the transformative power of these genetically modified bacteria, scientists may uncover novel avenues to combat a diverse range of afflictions, ultimately reshaping the landscape of medical interventions and revolutionizing patient outcomes.
As Maria Lluch-Senar and her dedicated team march steadfastly towards clinical trials, they lay the groundwork for future treatments that hold the promise of combatting human pneumonia. Their unwavering commitment extends beyond a single disease, with aspirations to revolutionize the management of diverse respiratory ailments such as lung cancer and asthma. Through their tireless efforts, they strive to transform these remarkable scientific advancements into tangible solutions that will make a tangible difference in the lives of countless individuals.
While the journey towards widespread therapeutic application of engineered bacteria remains arduous, this study serves as a pivotal milestone in the realm of bacterial engineering, offering a tantalizing glimpse into a future where personalized, bacteria-based treatments reign supreme.
Google News | Telegram